Bacteria in Recalled Eye Drops ‘Artificial Tears Distributed by EzriCazre’ Linked to Cases of Vision Loss, Surgical Removal of Eyeballs
Are you familliar with Recalled Eye Drops?
A rare strain of bacteria found in recalled eye drops ‘Artificial Tears distributed by EzriCazre’ has been linked to dozens of infections, as well as cases of vision loss, surgical removal of eyeballs and one death.
Global Pharma Healthcare’s Artificial Tears Lubricant Eye Drops, distributed by EzriCare and Delsam Pharma, were first recalled in early February.
In an update this week, the US Centers for Disease Control and Prevention identified 68 patients in 16 states with infections of a rare strain of drug-resistant Pseudomonas aeruginosa that had never before been reported in the United States. Most patients reported using artificial tears, the CDC said. Although patients reported using different brands, EzriCare Artificial Tears was the brand most commonly reported.
Reported adverse events as of March 14 include infections of the cornea, bloodstream, respiratory and urinary tract. There are eight reports of lost vision and four reports of surgically removed eyeballs. It was previously reported that one person has died.
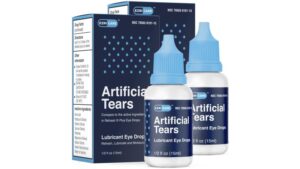
The US Food and Drug Administration and CDC have urged consumers to stop using the recalled eye drop products.
“Patients who have used EzriCare or Delsam Pharma’s artificial tears and who have signs or symptoms of an eye infection should seek medical care immediately,” the CDC said. Symptoms can include yellow, green or clear discharge from the eye; eye pain or discomfort; redness of the eye or eyelid; feeling like something is in the eye; increased light sensitivity; and blurry vision.
Global Pharma initiated a voluntary recall last month, and FDA recommended the recall due to manufacturing violations, including lack of appropriate microbial testing and being packaged in multi-use bottles without adequate preservatives.
In addition to Artificial Tears, FDA recommended on February 22 that Global Pharma recall Delsam Pharma’s Artificial Eye Ointment over concerns of bacterial contamination, which the company agreed to.
The company did not respond to CNN’s request for comment on Friday.
More eye drop recalls
More eye drop recalls have also been announced recently, although they are not linked to adverse events so far.

Pharmedica USA is recalling two lots of anti-inflammatory Purely Soothing 15% MSM Drops due “to non-sterility,” according to the March 3 FDA announcement. The company said it had not received any reports of adverse events or illness related to the product.
The company is advising consumers to stop using the eye drops immediately and return them to the place of purchase. Consumers with questions about the recall can call Pharmedica USA at 1-623-698-1752, provided on the FDA website.
Apotex is recalling six lots of Brimonidine Tartrate Ophthalmic Solution 0.15%, prescription eye drops used to treat open-angle glaucoma or ocular hypertension. The company says the recall is out of “an abundance of caution” due to cracks in some bottle caps that may impact sterility and lead to adverse events.
Article credit “www.cnn.com”